Patient Focused.

We offer a portfolio of allograft solutions that addresses complex chronic wounds. Many of our grafts are designed to repair soft tissue including diabetic foot ulcers, venous leg ulcers, sacral ulcers, and tunneling wounds. Our allografts are supported by published research demonstrating efficacy for mitigating, repairing, and reconstructing even the most challenging wounds. Our goal is not simply to repair damaged tissue, we seek to improve the patient’s overall quality of life through the gift of donation.
View Our Tissue & Product Catalog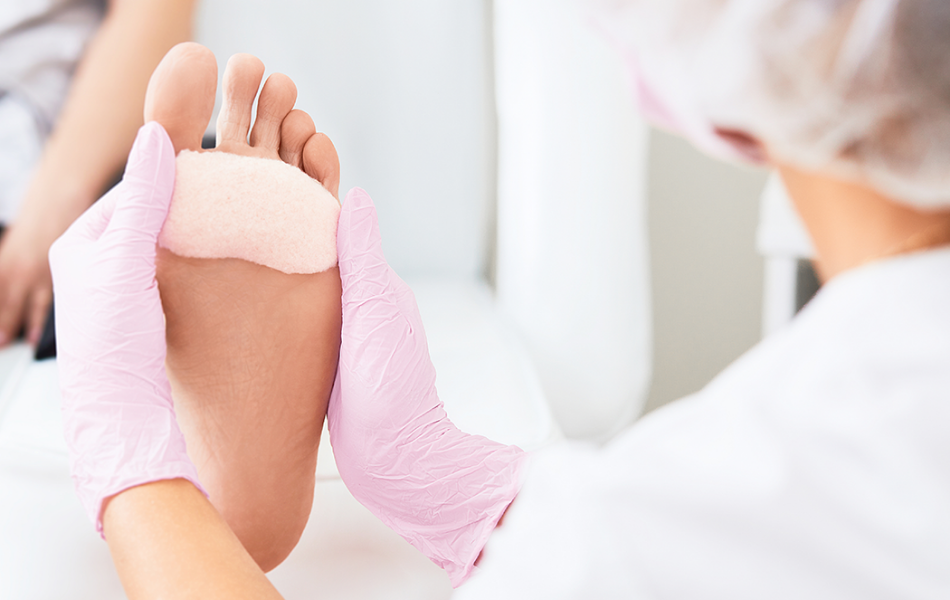
Chronic Foot & Leg Ulcers
Chronic foot and leg ulcers can be especially hard to treat and place a tremendous burden on society, not only in terms of patient quality of life but in associated medical costs. Our proven allograft solutions are cost-effective and deliver closure and better outcomes for patients with complex wounds.
Contact an Expert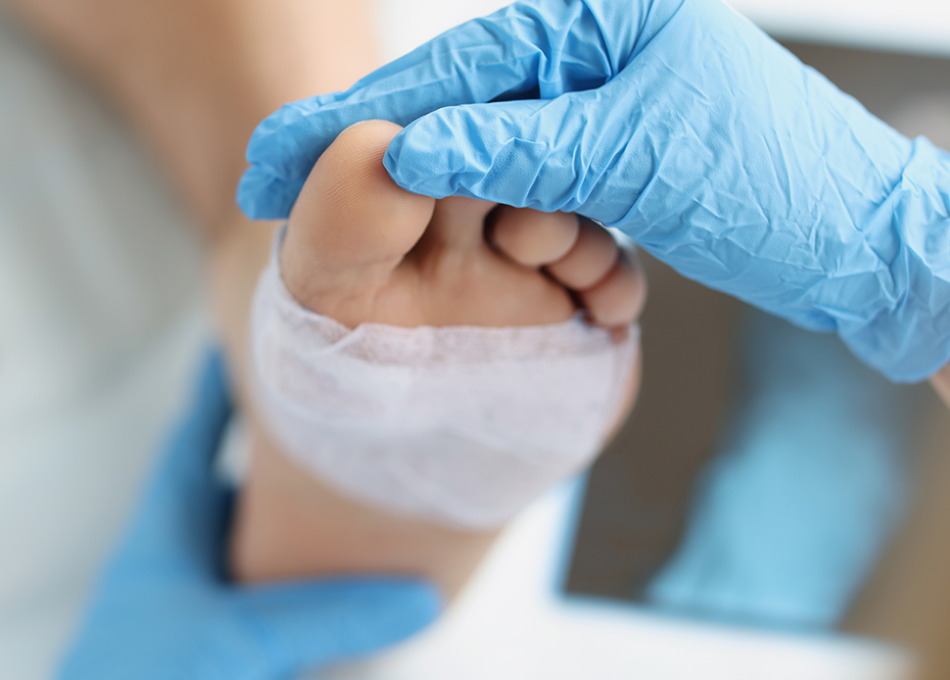
Plantar Reconstruction
Soft tissue defects of the feet are challenging to address. Restoring the integrity and function of the sole following injury, disease, or the excision of damaged tissue requires solutions that can withstand weight-bearing pressures while ensuring complete integration. Our advanced allograft solutions provide needed cover for plantar defects and restore damaged soft tissue. Their structural integrity and biological compatibility can help ensure better outcomes for even the most difficult wounds.
Contact an Expert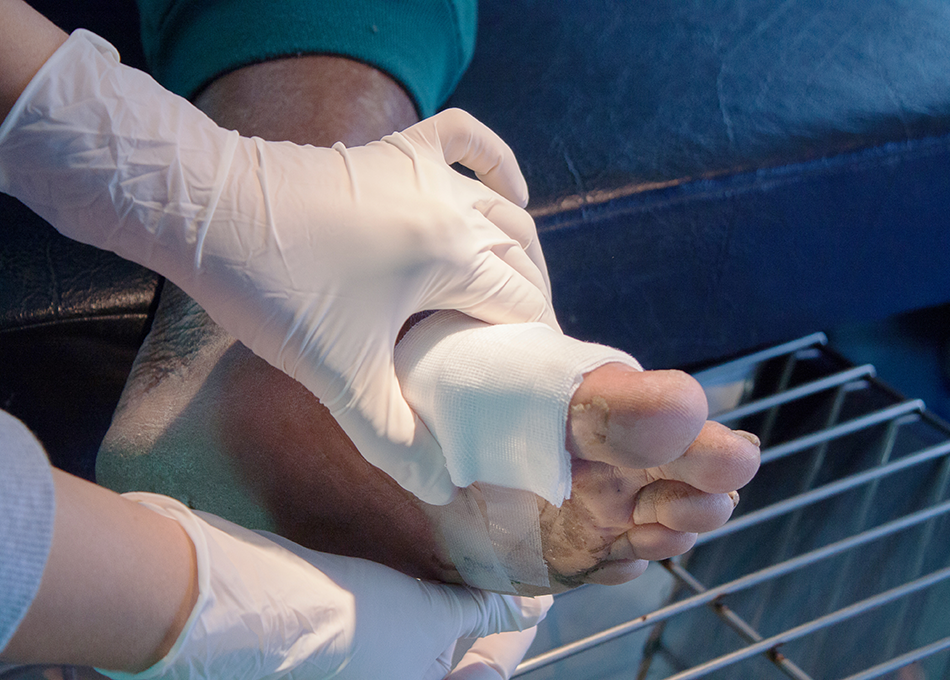
Pressure Ulcers
Tissue damage caused by long-term pressure can range in severity from mild dermal breakdown to deep wounds that expose the underlying layers of muscle and bone. We offer a variety of allograft solutions processed to restore soft tissue structure and enhance integration. Our aseptic processing methodology ensures the physical integrity of each graft, along with the preservation of its natural biological factors.
Contact an ExpertInquiries
Interested in learning more? Contact a representative today.




